KEEP UP WITH OUR DAILY AND WEEKLY NEWSLETTERS
sized at 35mm, the film cartridge can fit into a pocket, small bag, or even hang as an accessory.
connections: +540
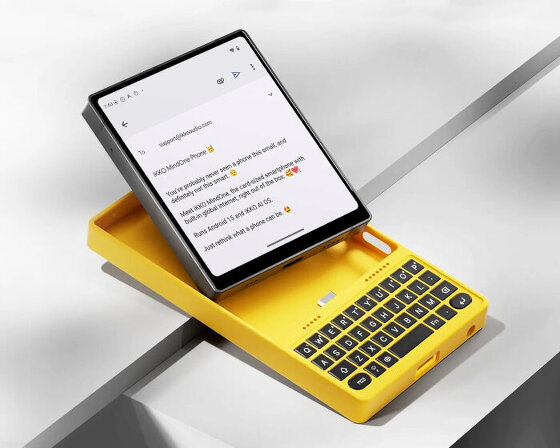
a modular device built around a physical keyboard, the case introduces a full QWERTY typing tool that attaches magnetically.
connections: +480
ori eliminates ribs, fabric, and typical failure points, creating an entirely new category of personal weather device.
connections: 36
the concept trike was inspired by a vision of getting around airports more efficiently.
connections: +430